XOPENEX HFA® (levalbuterol tartrate)
CLINICAL EFFICACY
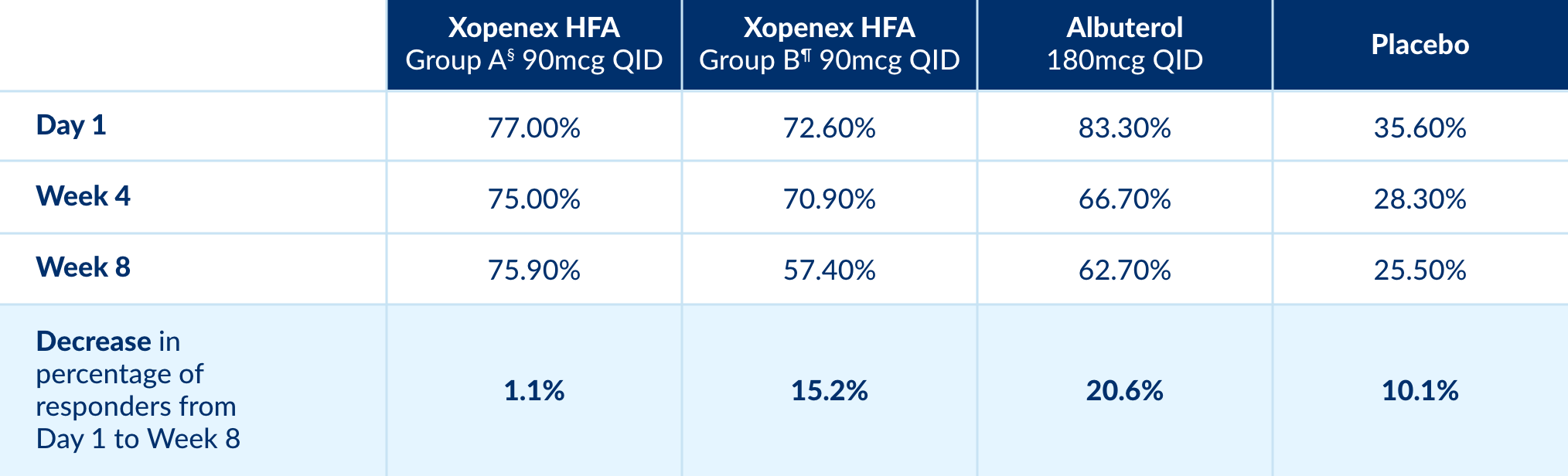
Xopenex HFA delivers high clinical response rates across treatment populations.1*
* Clinical response defined as a change in FEV1 greater than 15% from pre-dose1
Clinical response observed in
Patients continue to respond to treatment with Xopenex HFA far beyond day 1.1
Xopenex HFA demonstrated a sustained rate of response over time1
Percentage of Adolescent and Adult Responders1‡:
‡ Short acting beta 2 agonists were
administered 2 puffs QID over the 8-week duration of the study
§ Group A - Xopenex HFA manufactured at
Site A
¶ Group B - Xopenex HFA manufactured at
Site B
Xopenex HFA delivers needed bronchodilation with only the R-isomer of albuterol2,3
Xopenex HFA is well-tolerated, with a low incidence of beta-mediated
side effects2
Therapeutic activity of albuterol comes from the r-isomer; the
s-isomer may have pro-inflammatory effects3
Read Less ![]()
INDICATION
XOPENEX HFA® (levalbuterol tartrate) Inhalation Aerosol is
indicated for the treatment or prevention of bronchospasm in adults, adolescents, and children 4
years of age and older with reversible obstructive airway disease.
IMPORTANT SAFETY INFORMATION
Contraindication
XOPENEX HFA is contraindicated in patients with a history of hypersensitivity
to levalbuterol, racemic albuterol, or any other component of XOPENEX HFA Inhalation Aerosol.
Warnings and Precautions
- XOPENEX HFA and other β-agonists can cause paradoxical bronchospasm, which may be life threatening. Discontinue XOPENEX HFA immediately and treat with alternative therapy.
- Need for more doses of XOPENEX HFA than usual may be a sign of deterioration of asthma and requires reevaluation of treatment.
- XOPENEX HFA is not a substitute for corticosteroids.
- Cardiovascular effects may occur. Consider discontinuation of XOPENEX HFA if these effects occur. Use with caution in patients with underlying cardiovascular disorders.
- Excessive use may be fatal. Do not exceed recommended dose.
- Immediate hypersensitivity reactions may occur. Discontinue XOPENEX HFA immediately.
- Hypokalemia and changes in blood glucose may occur.
Adverse Reactions
- In patients aged 4 to 11 years, the most common adverse events (occurring in ≥2% of patients receiving XOPENEX HFA and more frequently than patients receiving placebo) were vomiting, accidental injury, pharyngitis, and bronchitis.
- In patients 12 years and older, the most common adverse events (occurring in ≥2% of patients receiving XOPENEX HFA and more frequently than patients receiving placebo) were asthma, pharyngitis, rhinitis, pain, and dizziness.
For more information about XOPENEX HFA (levalbuterol tartrate) Inhalation
Aerosol, please see the full Prescribing Information.
To report SUSPECTED ADVERSE REACTIONS, contact Lupin
Pharmaceuticals at +1 866-587-4617 or at drugsafety@lupin.com or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
REFERENCES: